More breakthroughs today; fewer outbreaks tomorrow. Our cutting-edge diagnostic technology and real-time disease surveillance solutions are empowering our clients and collaborators to prepare for the next pandemic.

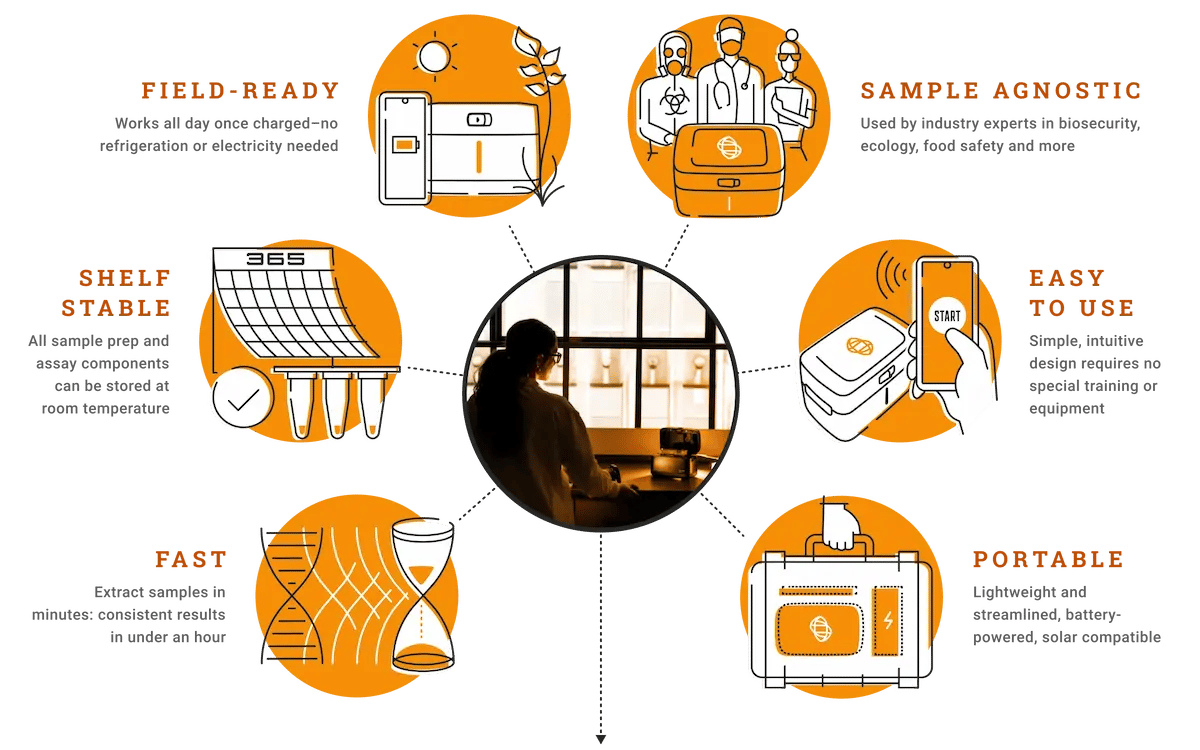
We understand the critical importance of rapid and accurate testing for controlling the spread of viruses and protecting global health. Our user-friendly and ultra-portable platform can be used in a variety of settings to help avoid the need for drastic measures, such as lockdowns and quarantines, which have significant economic and social impacts.
The speed, accuracy, and sensitivity of our real-time PCR and isothermal molecular tests and fully-automated mobile platforms provides reliable results and peace of mind when health and safety are paramount.

At the start of the COVID-19 pandemic, we received one of the first FDA emergency use authorizations. We manufactured and shipped thousands of thermocyclers and millions of tests. Our testing services subsidiary expanded from one CLIA high-complexity lab and one employee in one city to four CLIA high-complexity labs and 350 employees across eight major cities. COVID-19 accelerated the deployment of our vision for decentralized diagnostics and real-time disease surveillance. And we will use the lessons we learned and the technologies we developed to prepare for and contain the next global health emergency.











401 North Broad St Suite 222 Philadelphia, PA 19108